Due to its fine geometry and excellent physical properties, the superlattice has attracted people's extensive interest and attention, and has opened up new fields for finding new materials and new light sources. Molecular beam epitaxy (MBE) as an atomic-scale processing technology enables precise control of growth thickness, structure, and composition, and is the most advantageous tool for preparing superlattices. However, it also faces many problems, such as expensive preparation facilities, complicated operating procedures, harsh growth conditions, and strong dependence on substrates, which greatly limit the development and application of superlattices. Therefore, the search for new superlattice growth mechanisms and methods is one of the current goals of materials science and technology. Recently, the Institute of Physics, Chinese Academy of Sciences/Beijing National Laboratory for Condensed Matter Physics (CPC) Wang Weihua's research team cooperated with the Gulin Research Group and discovered that Pd-based and other bulk amorphous alloys (also known as metallic glass) are lower than glass. Annealing at the temperature of the transition temperature (Tg) condition, a superlattice-like structure can be stably grown on the free surface of the metallic glass.
Amorphous alloys are alloy materials formed by high-temperature melts through rapid cooling techniques and are kinetically called "frozen liquids." As a relatively simple, isotropic, glassy material with a topological structure, amorphous alloys are an ideal system for studying the intrinsic properties of glass. At high temperatures, the viscosity of the metal liquid is very small (only about 10-4 Pa s) and the overall macroscopic flow behavior occurs. As the temperature decreases, when the states from the ergodic to the vitreous state of the liquid undergo a break-ergodic transition (i.e., the glass transition process, the viscosity at the glass transition temperature is about 1012 Pas), this This kind of macro-flow behavior will be frozen. However, the freezing of this macro-flow is not "complete." The previous experimental study by the research group showed that [Applied Physics Letters 107, 141606 (2015)]: In the temperature zone below the glass transition temperature point, the free surface of the amorphous alloy is still in a liquid-like state. This is due to the fact that the free surface atoms of the metal glass are weaker than the body due to the “dynamical limitation†of neighboring atoms from the surrounding, making its surface diffusion rate about 105 times faster than the body's atoms, which provides a highly ordered crystal self-organized growth. Excellent environmental conditions. The research group chose Pd40Ni10Cu30P20 alloy system with excellent anti-oxidation and glass forming ability. Using a long isothermal annealing method at a temperature lower than Tg, a large-area, large-lattice period, class-like ultra-structure was achieved on the disordered surface of metallic glass. Stable growth of the lattice modulation structure. Based on high-resolution transmission and spherical electron microscope observations and X-ray photoelectron spectroscopy analysis, the microscopic mechanism was explored and revealed.
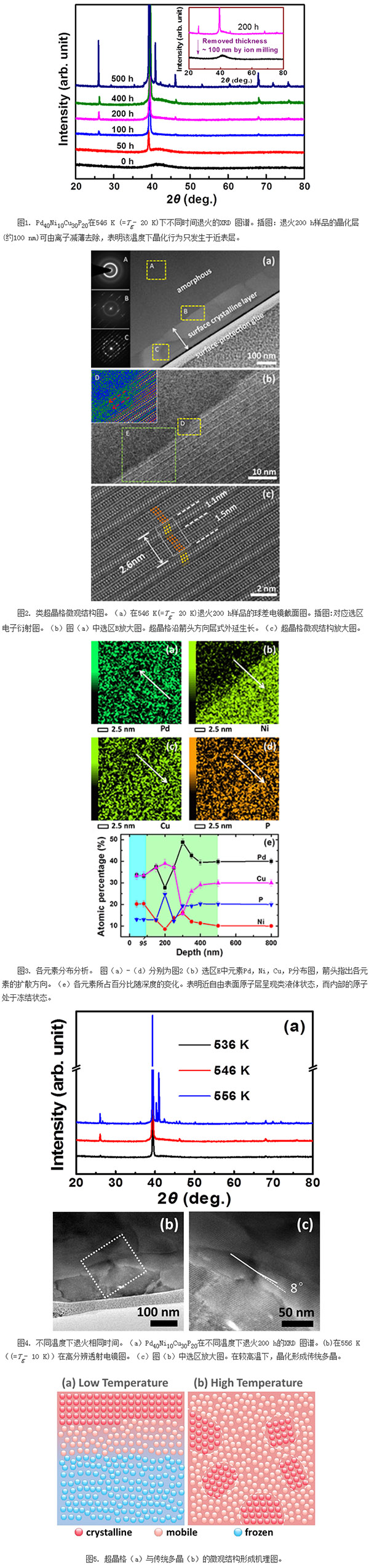
It was found that, unlike the deposition of a superlattice structure on a single-crystal substrate in a conventional molecular beam epitaxy method, the crystallization behavior of the metallic glass begins at its atomic-scale disorder surface at 546 K (Tg-20K). 1); As the annealing time is extended, a superlattice-like structure with a certain thickness and a highly ordered structure is grown stably and continuously on the surface (Fig. 2); elemental analysis based on measurements at different depths shows that the surface atom and the diffusion rate of the atoms in the body The decoupling mechanism is the origin of the microstructure of the crystal (Figure 3); by changing the growth conditions, it was found that at higher temperatures (Tg-10K or more), the amorphous alloy system gradually changes from surface crystallization to in-vivo crystal with increasing temperature. The formation and formation of a conventional polycrystal (Fig. 4) shows that the formation of the surface superlattice structure can be achieved near the Tg point by simply controlling the temperature (Fig. 5). Due to the simple growth conditions and without the need for substrate assistance, the research work greatly simplifies the preparation process of the superlattice, reduces the manufacturing cost, and provides new ideas and new methods for the controlled growth of the superlattice structure. The related results were recently published in the Physical Review Letters [Physical Review Letter 118, 016101 (2017)] with the topic Fast surface dynamics of metallic glass enable superlattice-like nanostructure growth.
The research work was funded by the National Natural Science Foundation Soft Magnetic Group Project (51571209), the “973†Project (project approval number 2015CB856800), and the key projects of the Frontier Bureau of the Chinese Academy of Sciences.
Chrome Plated Brass Taps,Chrome Plated Brass Tap,Chrome Plated Brass Tubing,Chrome Plated Brass Pipe
AS-SUR INDUSTRIAL VALVE CO., LTD. , https://www.assur-valve.com