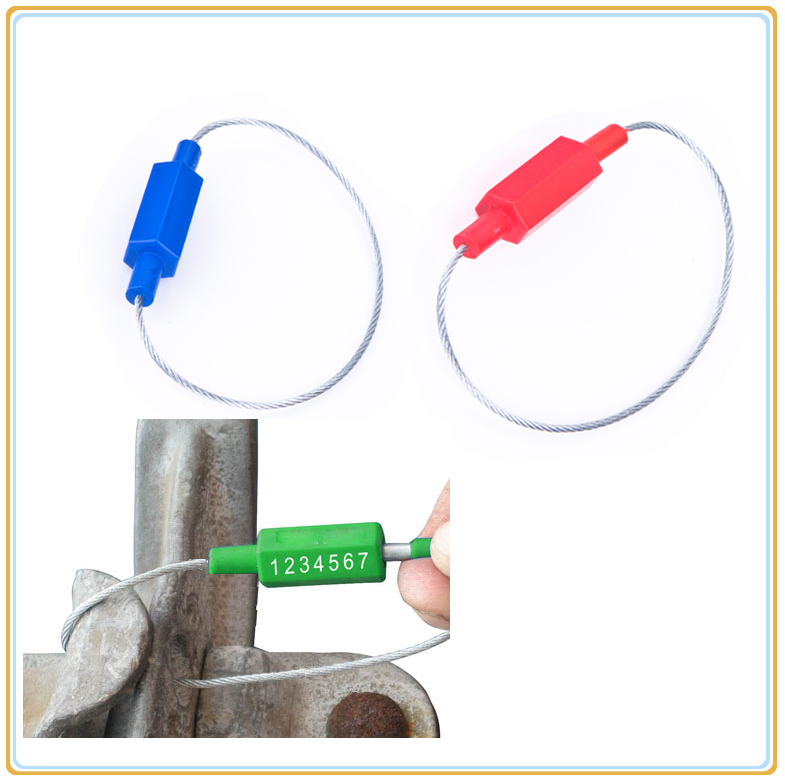
| Specification: | Lock Material: Â | Zine alloy wrapped ABS |
| Wire Material: | Low carbon steel | |
| Tensile Strength: Â | more than 784N | |
| Total Length: | 230mm (Standard) | |
| Cable Diameter: | 1.80mm | |
| Flag Dimension: | 7.5*12*27mm | |
| Length | Customised | |
| Operation Temperature: | -40º Cto -110º C(-40° F to 230° F) | |
| Printing: | Hot stamp custom company name, logo and sequential numbers | |
| Bar code decoration is available |  | |
| Colour: | Standard Colours(Anodized) | Green, Red, Blue, Yellow, White, Orange |
| Other colors | Available | |
| Packaging: | Standard Packaging | 100pcs/per bundel |
| 1, 000pcs/per Carton | ||
| Carton Dimension | 39.5cm× 28.5cm× 20cm | |
| One Seal Weight: | 15.8g | |
| Delivery Time: | As customer' s requirement | |
| Sample Time: | 2-3 working days after confirm orders. | |


Â
The COVID-19 Antigen Saliva Test kit is a lateral flow immunoassay intended for the preliminary screening and qualitative detection of nucleocapsid protein antigen from severe acuterespiratorysyndrome coronavirus SARS-CoV-2which causesthe Coronavirusdisease(COVID-19),in direct saliva sample from individuals suspected of coVD-19 by their healthcare provider within the first seven days of symptom onset.The COVID-19 Antigen Saliva Test kit is for professional use only and is intended to be used as an aid in the diagnosis of SARS CoV-2 infection.
SARS-CoV-2is anenvelopedsingle-stranded RNAvirus ofthe Baenus COVD-19 is an acute respiratory infectious disease. People are generally susceptible.Currently. the patients infected bytheSARS-CoV2are the main source ofinfection:asvmptomatic infected peope car also be an infectious source Based on thecurrentepidemiologica investigation, the incubation period is 1to 14 daysmostly 3to 7 days The main manifestations include feverfatigue and drycough.Nasa congestion, runny nosesore throatmyalgia and diarhea are found in a few cases.
Results are for the identification of SARS-CoV-2 nuceocapsid protein antigen. The SARS-CoV-2 antigen is generally detectable in upper respiratory specimens during the acute phase ofinfectionPositive results indicate the presence of SARS-CoV-2viralantigenshowever cinicai corelation with patient history and other diaanostic information is key to determine status of infection.Positive results don't preclude bacterialinfection or co-infection with other yiruses.eaatveresuts To patients with symptom onset for more than seven days should be treated as presumptive and confirmation with a molecular assayand patient management may be performed according to local regulations as needed.
saliva antigen rapid test kit,antigen test saliva,antigen saliva test kit,rapid antigen saliva
Yong Yue Medical Technology(Kunshan) Co.,Ltd , https://www.yonyue.com